Health Today
Researchers discover link between inflammation and spread of breast cancer
 Cancer is lethal because it spreads, or metastasizes, and curing cancer depends on knowing just how this process works. Cornell biomedical engineers have uncovered a groundbreaking link between the body's natural inflammatory response, and how malignant breast cancer cells use the bloodstream to metastasize.
Cancer is lethal because it spreads, or metastasizes, and curing cancer depends on knowing just how this process works. Cornell biomedical engineers have uncovered a groundbreaking link between the body's natural inflammatory response, and how malignant breast cancer cells use the bloodstream to metastasize.
Their work, published online Jan. 23 in the open-access, peer-reviewed journal PLOS One, contends that pro-inflammatory signaling molecules in blood called cytokines constitute a "switch" that induces the mechanism by which breast cancer cells "roll" and adhere to the blood vessel surface. The cancer cells eventually stick to and infiltrate the vessel.
The paper's first author is Yue Geng, graduate student in the field of biomedical engineering, working in the lab of Michael King, professor of biomedical engineering, who is the paper's senior author.
King's lab members study how cancer cells spread through the bloodstream by interacting with the blood vessel wall, called the endothelium. Cancer cells have small molecules, called ligands, on their surfaces that find specific receptor sites on the endothelium, called selectins. The ligand-selectin interaction causes the cells to literally "roll" on the endothelium, slowing down and eventually adhering to the blood vessel, in a process called the metastatic cascade. This mechanism is identical to how white blood cells infiltrate blood vessels to reach the site of inflammation.
King lab
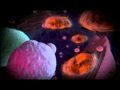
An illustration of the positive feedback loop of the activation and maintenance of the adhesive phenotypic switch induced by pro-inflammatory cytokines. Tumor cells encounter cytokines IL-6 and TNF-alpha secreted by tumor-promoting immune cells in the microenvironment. Cytokine-conditioned tumor cells form aggregates, which release more cytokines, inducing more tumor cells nearby to be able to roll on the endothelium, leading to metastasis.
Cancer has long been associated with inflammation -- the body's natural defense mechanism -- and now the researchers have demonstrated a definitive link. They found that the presence of pro-inflammatory molecules -- the cytokines IL-6 and TNF-alpha -- enable the malignant, hormone therapy-resistant breast cancer cells used in the study to adhere to the endothelial wall, leading to metastasis.
Tumor cells first encounter IL-6 and TNF-alpha in the primary tumor's microenvironment (before the cancer has spread). These cytokines induce proliferation and aggregation of cancer cells, triggering other cancer cells to secrete more cytokines, resulting in a positive feedback loop.
The King lab has developed a flow chamber that mimics inflamed endothelium and has used this to investigate the metastatic cascade on the bench top. In investigating the adhesive behavior of a particularly metastatic cell line, Geng and colleagues discovered unexpectedly that these cells were unable to interact with selectins -- a key step in the metastatic cascade.
The bioengineers went on to design several different cell culture setups to culture cancer cells with human plasma, IL-6 and TNF-alpha to test their hypotheses that inflammatory molecules in blood may induce adhesion capability. All of them promoted breast cancer cell metastatic behavior.
They also used more sophisticated, 3-D tumor spheroids, which are more physiologically accurate, to confirm their results. In fact, the 3-D spheroid tumor cells exhibited the most significant increase in the interaction between the cancer cells and the blood vessel. They also treated some of the samples with a known anti-inflammatory drug called Metformin, which blocks IL-6, and they found that these samples were not able to metastasize -- further underscoring their results.
Improving cancer treatment to fight metastasis via the bloodstream will depend on undoing this roll-and-stick mechanism of cancer cells, Geng said. The Cornell research could form the basis for immunotherapies to block the ligand-selectin binding of cancer cells, by first counteracting the inflammatory cytokines that, it seems, set the whole process in motion.
The research was funded by the National Cancer Institute via the Center on the Microenvironment and Metastasis, and a National Science Foundation Graduate Research Fellowship.
View Source Article Stress, depression may affect cancer survival
Stress, depression may affect cancer survival Defending Against Disease With an Anti-Inflammation Lifestyle
Defending Against Disease With an Anti-Inflammation Lifestyle Increased cardiovascular risk in HIV-infected patients may relate to arterial inflammation
Increased cardiovascular risk in HIV-infected patients may relate to arterial inflammation Inflammation Gone Wild Can Lead To Macular Degeneration
Inflammation Gone Wild Can Lead To Macular Degeneration Chronic Inflammation and Late-Life Decline
Chronic Inflammation and Late-Life Decline INFLAMACIÓN CRÓNICA: EL PROBLEMA DE SALUD DEL CUAL NO HAS ESCUCHADO
INFLAMACIÓN CRÓNICA: EL PROBLEMA DE SALUD DEL CUAL NO HAS ESCUCHADO Dwelling On Stressful Events Can Increase Inflammation in the Body, Study Finds
Dwelling On Stressful Events Can Increase Inflammation in the Body, Study Finds Why Cancer and Inflammation?
Why Cancer and Inflammation? Chronic Inflammation: Reduce It to Protect Your Health
Chronic Inflammation: Reduce It to Protect Your Health Inflammation, Stress and Metabolic Diseases
Inflammation, Stress and Metabolic Diseases Researchers discover link between inflammation and spread of breast cancer
Researchers discover link between inflammation and spread of breast cancer The New Science Behind America's Deadliest Diseases
The New Science Behind America's Deadliest Diseases Foods That Fight Inflammation — And Why You Need Them
Foods That Fight Inflammation — And Why You Need Them MIT confirms link between inflammation, cancer
MIT confirms link between inflammation, cancer Is Chronic Inflammation the Key to Unlocking the Mysteries of Cancer?
Is Chronic Inflammation the Key to Unlocking the Mysteries of Cancer? What Causes Chronic Inflammation?
What Causes Chronic Inflammation? Chronic Inflammation: Reduce It to Protect Your Health
Chronic Inflammation: Reduce It to Protect Your Health Markers of Inflammation and Cardiovascular Disease
Markers of Inflammation and Cardiovascular Disease FAMILY HEALTH GUIDE
FAMILY HEALTH GUIDE The Anti-Inflammation Diet
The Anti-Inflammation Diet